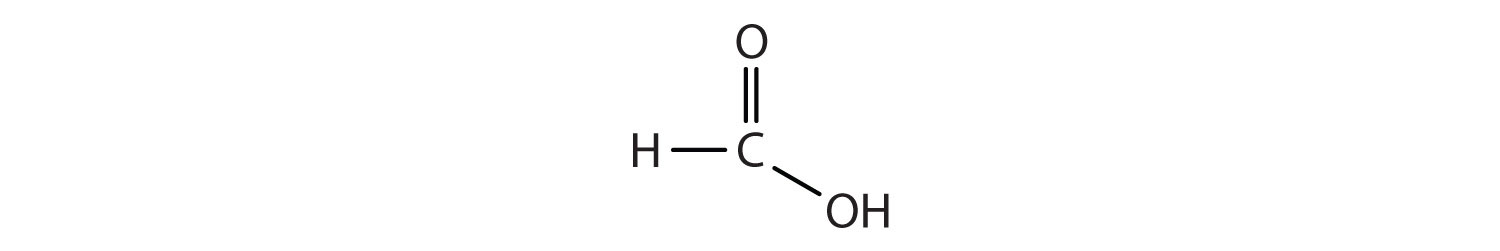
C Double Bond O Single Bond H

The simple view of the bonding in carbon oxygen double bonds.
C double bond o single bond h. The polarity of oxygen also makes the alpha hydrogens of carbonyl compounds much more acidic roughly 10 30 times more acidic than typical sp 3 c h bonds such as those in methane for example the pk a values of acetaldehyde and acetone are 16 7 and 19 respectively while the pk a value of methane is extrapolated to be approximately 50. This is because a carbonyl is in tautomeric resonance. Single bond double bond and triple bond. We are going to look at the bonding in methanal but it would equally apply to any other compound containing c o.
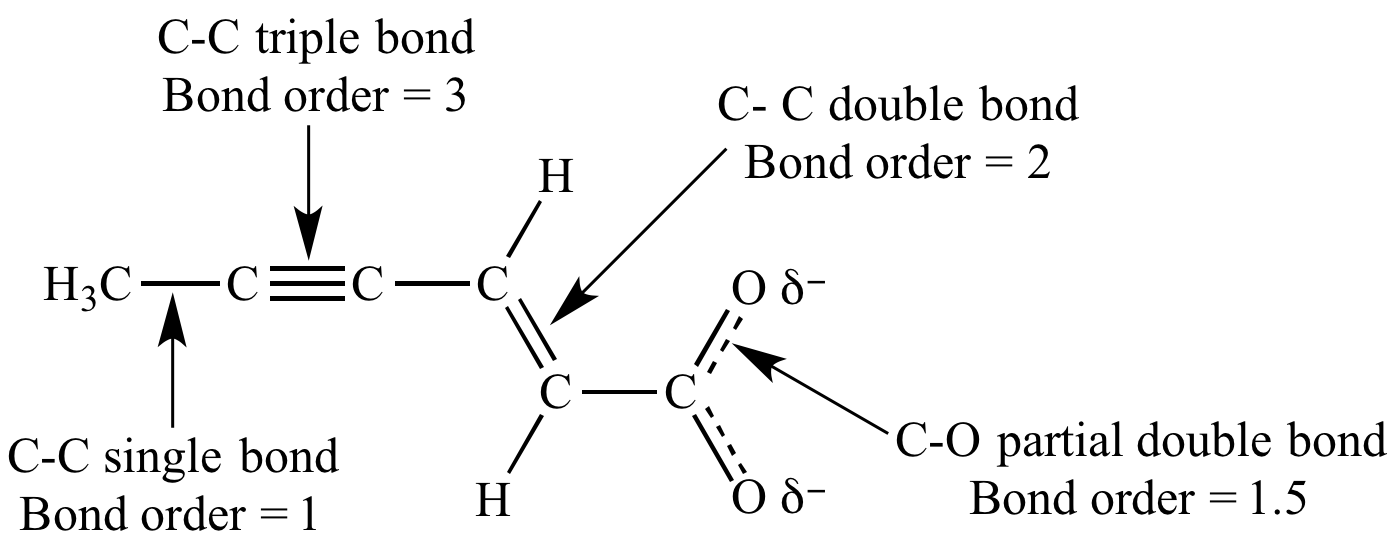
Shortened single bonds are found with carboxylic acids 136 pm due to partial double bond character and elongated bonds are found in epoxides 147 pm. The common example of a molecule with a double bond is ethylene. One of the ways of forming bonds is sharing of electron to attain their nearest noble gas configuration. The simplest compound containing this group is methanal.
Where the carbon oxygen double bond c o occurs in organic compounds it is called a carbonyl group. This way of bonding is known as covalent bonding and this is shown mainly by non metals and h. Aldehydes and ketones contain a c o carbonyl group note that in condensed structural formulas the aldehyde group may be written as ch o or as cho i. The two atoms can combine in different ways.
In ethylene the double bond is between two carbon atoms. Electronegativities and bond lengths.