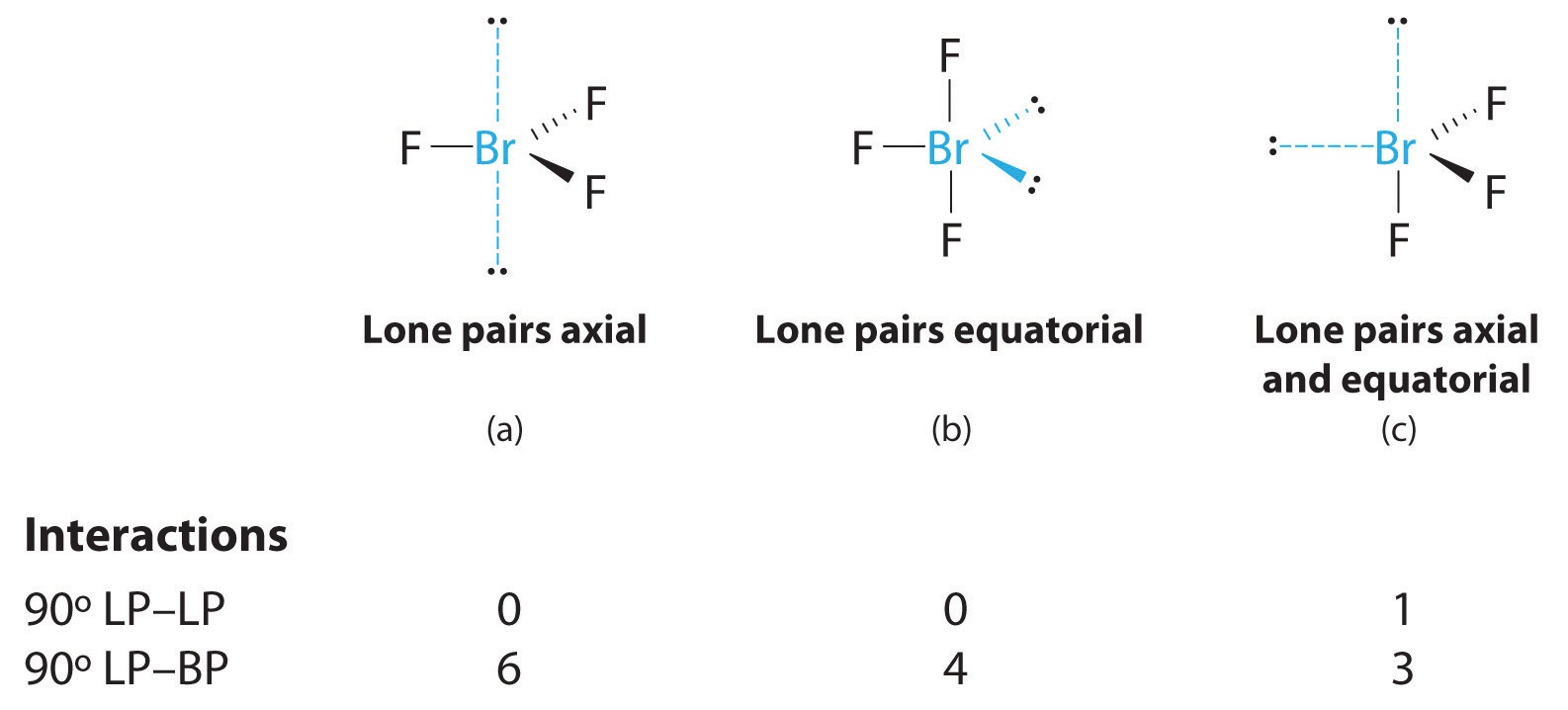
Lewis Structures Of Two Hf Molecules Interacting In A Stabilizing Geometry
Predict the local geometry for the nitrogen atom the two carbon atoms and the oxygen atom with a hydrogen atom attached.
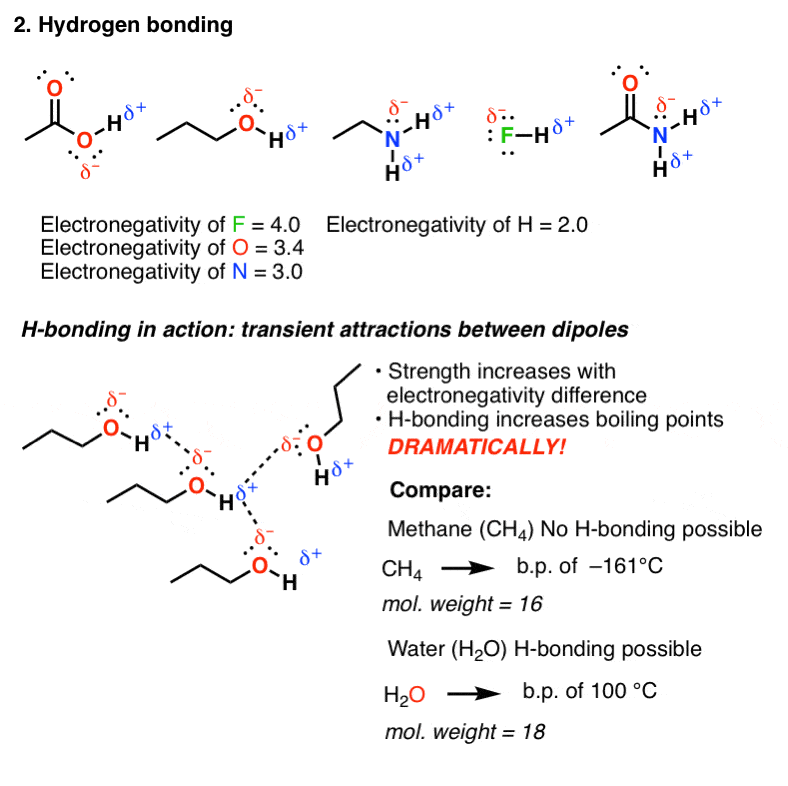
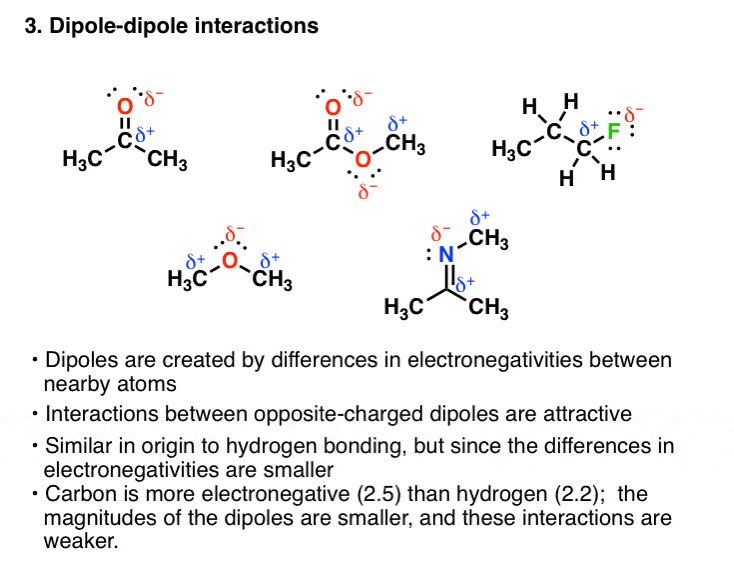
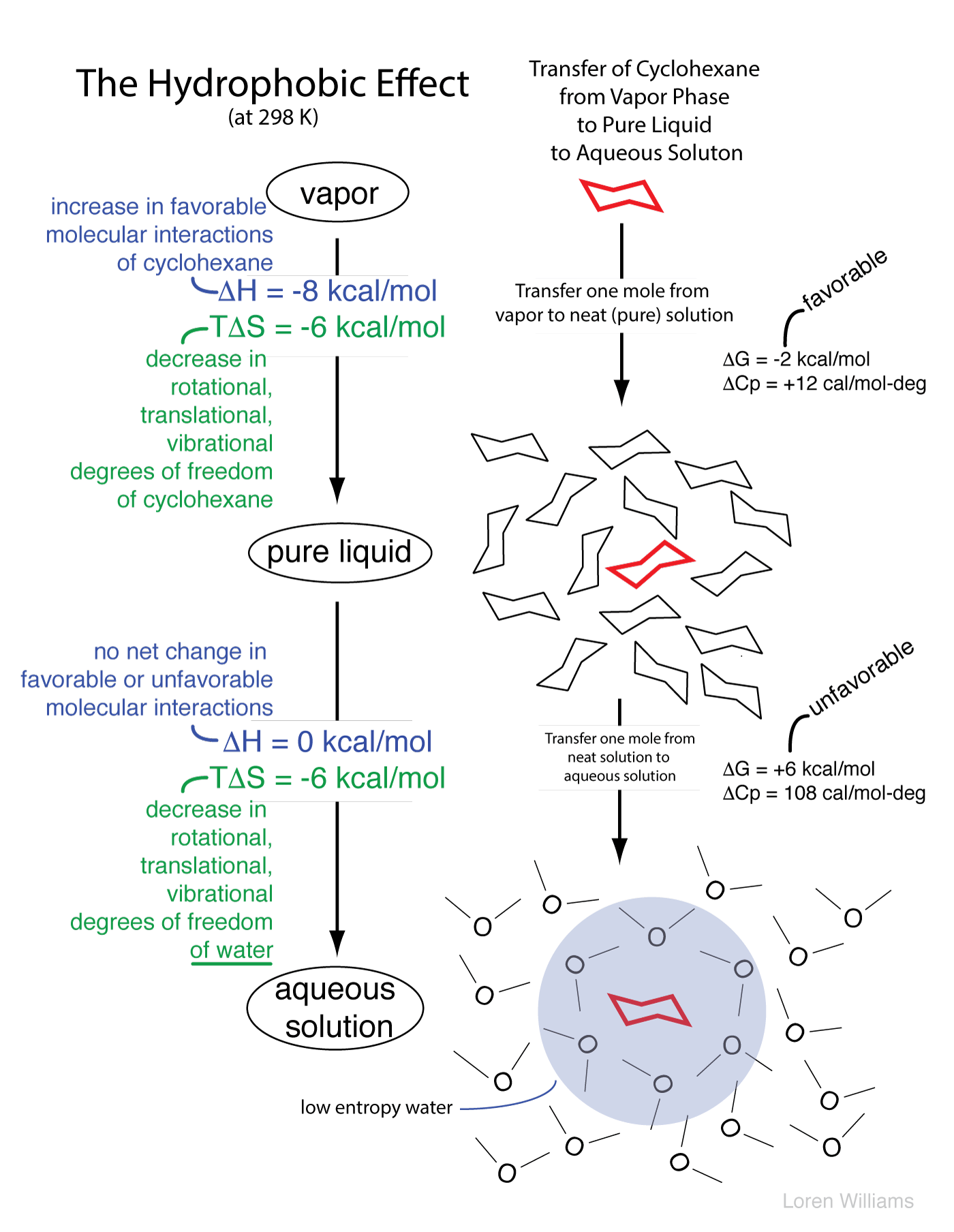
Lewis structures of two hf molecules interacting in a stabilizing geometry. Ammonia nh3 has the same types of imfs as hf. However molecular structure is actually three dimensional and it is important to be able to describe molecular bonds in terms of their distances angles and relative arrangements in space a bond angle is the angle between any two bonds that include a common atom usually measured in degrees. Nucleic acid tertiary structure is the three dimensional shape of a nucleic acid polymer. The lewis structure of hydrogen sulfide is easy to draw and understand.
How the molecule might react with other molecules. Rna and dna molecules are capable of diverse functions ranging from molecular recognition to catalysis such functions require a precise three dimensional tertiary structure while such structures are diverse and seemingly complex they are composed of recurring easily recognizable tertiary structure. The shape of a molecule. The key is to understand the steps and practice.
Circle the imf that gives rise to the very high boiling point in comparison to other hydrogen halides. Lewis structures are important to learn because they help us predict. Predicting structure in multicenter molecules. Thus far we have used two dimensional lewis structures to represent molecules.
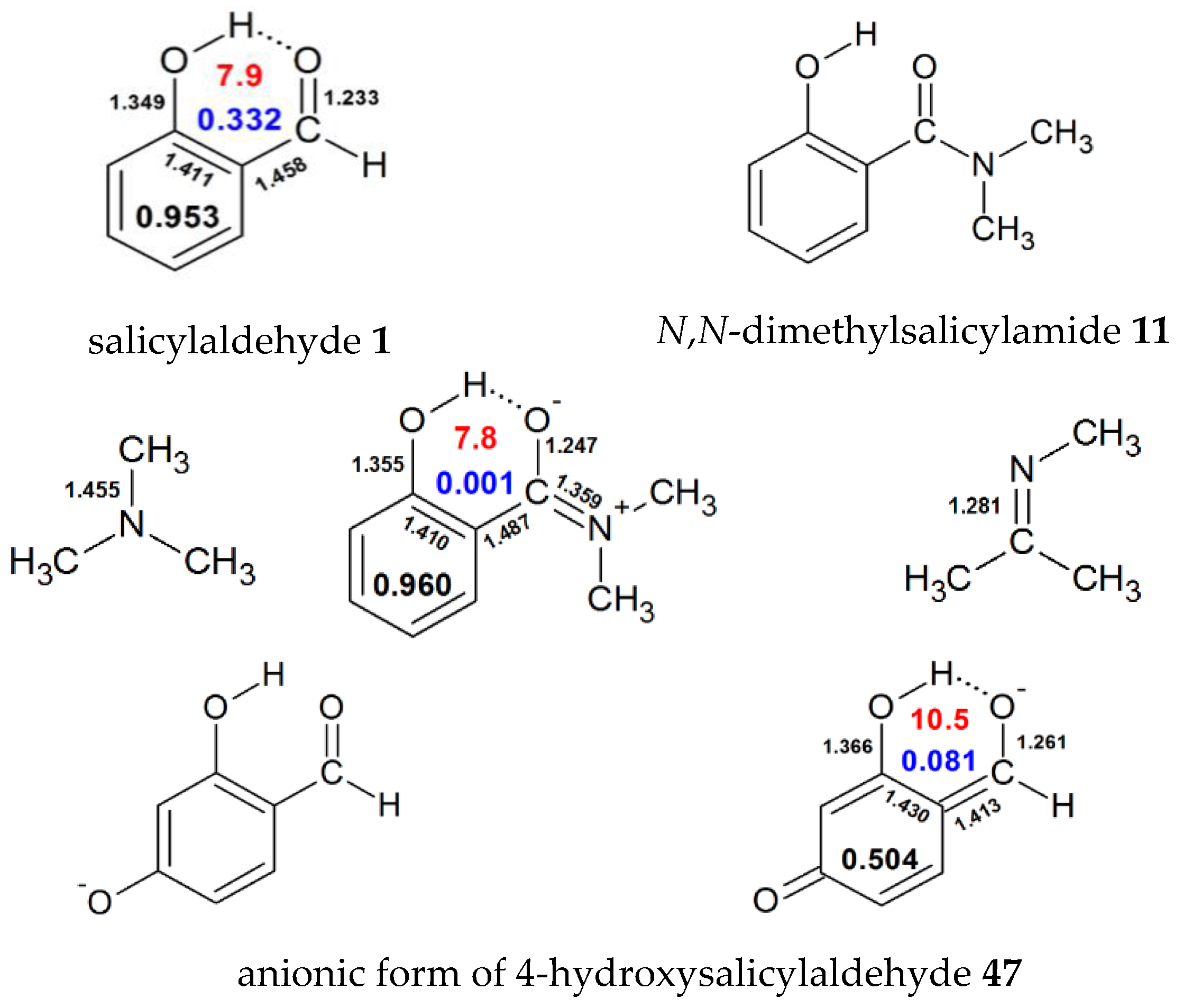
Thus the 12 h h interactions contribute 19 2 kcal mol 1 to the stabilization of the t d structure and the 18 such interactions contribute 13 2 kcal mol 1 or 0 8 kcal mol 1 per h h interaction to the t structure leading to a reduction in the stabilizing contribution from h h bonding in passage from the t d to the more stable t structure. Draw the lewis structures of two hf molecules interacting in a stabilizing geometry. Every chemistry student has to learn how to draw lewis dot structures. In this example we can draw two lewis structures that are energetically equivalent to each other that is they have the same types of bonds and the same types of formal charges on all of the structures both structures 2 and 3 must be used to represent the molecule s structure the actual molecule is an average of structures 2 and 3 which are called resonance structures.
The physical properties of the molecule like boiling point surface tension etc. Consider each central atom independently. Circle the imf that gives rise to the very high boiling point in comparison to other hydrogen halides. Ni3 lewis structure molecular geometry.
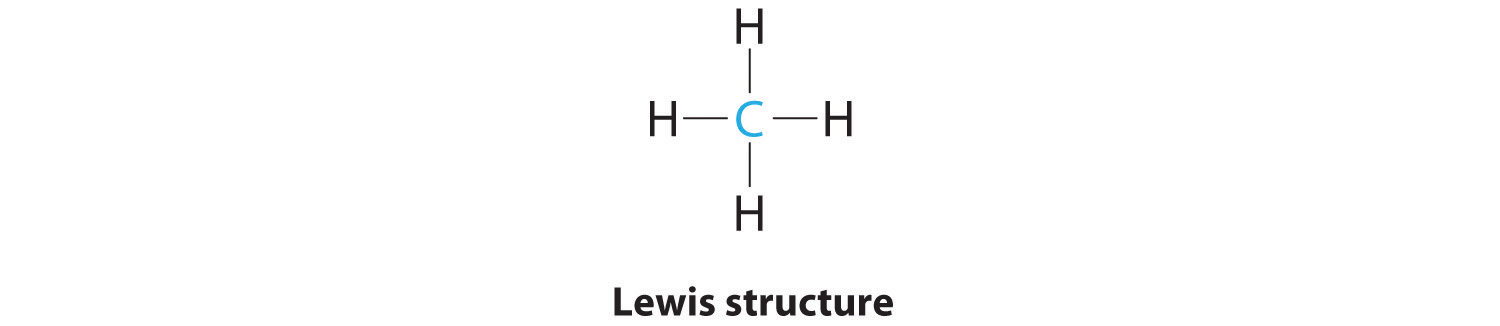
There are two atoms of hydrogen and a single atom of sulfur in the compound. The imfs have a lower effect on the boiling point of ammonia than if it were the case of water or hf. Each hydrogen atom has one only one electron which is also its valence electron and sulfur has six valence electrons.